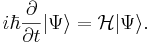Schrodinger was an unconventional man. Throughout his life he traveled with walking-boots and
rucksack and for this he had to face some difficulty in gaining entrance to the Solvay Conference for
Nobel laureates. Describing the incident Paul Dirac wrote: When he went to the Solvay Conferences in
Brussels, he would walk from the station to the hotel, carrying all his luggage in a rucksack and
looking so like a tramp that it needed a great deal of argument at the reception desk before he could
claim a room.
Schrodinger was born on August 12, 1887 in Vienna. His father Rudolf Schrodinger, who came
from a Bavarian family, which had come to Vienna generations ago, was a highly gifted man.
After studying chemistry at the Technical College in Vienna, Rudolf Schrodinger devoted himself
for years to Italian painting and then he decided to study botany. He published a series of research
papers on plant phylogeny.
Rudolf Schrodinger had inherited a small but profitable business manufacturing linoleum and oilcloth.
Schrodinger mother, Georgine Schrodinger (nee Bauer) was the daughter of Alexander Bauer, an
able analytical chemist and who became a professor of chemistry at the Technical College, Vienna.
Schrodinger was always grateful to his father for giving him a comfortable upbringing and a good
education. He described his father as a man of broad culture, a friend, teacher and inexhaustible
partner in conversation.
Schrodinger was taught by a private tutor at home until he entered the Akademisches Gymnasium in 1898.
He passed his matriculation examination in 1906. At the Gymnasium, Schrodinger was not only
attracted to scientific disciplines but also enjoyed studying grammar and German poetry. Talking
about his impression at the Gymnasium Schrodinger later said: I was a good student in all subjects,
loved mathematics and physics, but also the strict logic of the ancient grammars, hated only memorizing
incidental dates and facts. Of the German poets, I loved especially the dramatists, but hated the
pedantic dissection of their works. He was an outstanding student of his school.
He always stood first in his class. His intelligence was proverbial. One of his classmates commenting
on Schrodinger ability to grasp teachings in physics and mathematics said: Especially in physics and
mathematics, Schrodinger had a gift for understanding that allowed him, without any homework,
immediately and directly to comprehend all the material during the class hours and to apply it.
After the lecture…it was possible for (our professor) to call Schrodinger immediately to the
blackboard and to set him problems, which he solved with playful facility.
In 1906, Schrodinger joined the Vienna University. Here he mainly focused in the course of theoretical
physics given by Friedrich Hasenohrl, who was Boltzmann student and successor. Hasenhorl gave an
extended cycle of lectures on various fields of theoretical physics transmitting views of his teacher,
Boltzmann.
Schrodinger received his PhD in 1910. His dissertation was an experimental one. It was on
humidity as a source of error in electroscopes. The actual title of the dissertation
was
On the conduction of electricity on the surface of insulators in moist air. The work was
not very significant. The committee appointed for examining the work was not unanimous in
recommending him for the degree. After receiving his PhD, he undertook his voluntary military service.
After returning from military service in autumn 1911, he took up an appointment as an assistantship
in experimental physics at the University of Vienna. He was put in charge of the large practical
class for freshmen. Schrodinger had no love for experimental work but at the same time he valued the
experience. He felt that it taught him through direct observation what measuring means. He started
working in theoretical physics by applying Boltzmann-like statistical-mechanical concepts to magnetic
and other properties of bodies. The results were not very significant. However, based on his work
he could earn his advanced doctorate (Habilitation).
At the beginning of the First World War, Schrodinger was called up for active service. He was sent
to the Italian border. It was at the warfront that Schrodinger learned about Einstein general
theory of relativity and he immediately recognized its great importance. While in war field it was
not possible for Schrodinger to keep him fully abreast of the developments in theoretical physics.
However, he continued his theoretical work. He submitted a paper for his publication from his position
on the Italian front. In the spring of 1917, Schrodinger was transferred to Vienna, where he again
could start scientific work.
The First World War resulted in total collapse of the economy of Austria. It also ruined Schrodinger
family. Schrodinger had no option other than to seek a career in the wider German-language world of
Central Europe. Between spring 1920 and autumn 1921, Schrodinger took up successively academic
positions at the Jena University (as an assistant to Max Wien, Wilhelm Wien brother, at the
Stuttgart Technical University(extraordinary professor), the Breslau University (ordinary professor),
and finally at the University of Zurich, where he replaced von Laue. Soon after arriving at Zurich,
Schrodinger was diagnosed with suspected tuberculosis and he was sent to an alpine sanatorium in
Arosa to recover. While recuperating at Arosa, Schrodinger wrote one of his most important papers,
On a Remarkable Property of the Quantized Orbits of an Electron. At Zurich he stayed for
six years. This was his most productive and beautiful period of his professional life.
It was at Zurich that Schrodinger made his most important contributions. He first studied atomic
structure and then in 1924 he took up quantum statistics. However, the most important moment of his
professional career was when he came across Louis de Broglie work. On November 03, 1925,
Schrodinger wrote to Einstein: A few days ago I read with great interest the ingenious thesis
of Louis de Broglie, which I finally got hold of ... And then on 16th November he wrote:
I have been intensely concerned these days with Louis de Broglie ingenious theory.
It is extraordinarily exciting, but still has some very grave difficulties. After reading de Broglie
work Schrodinger began to think about explaining the movement of an electron in an atom as a
wave and eventually came out with a solution. He was not at all satisfied with the quantum
theory of the atom developed by Niels Bohr, who was not happy with the apparently arbitrary
nature of a good many of the quantum rules. Schrodinger did not like the generally accepted dual
description of atomic physics in terms of waves and particles. He eliminated the particle altogether
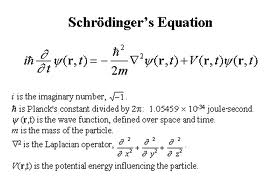
and replaced it with wave alone. His first step was to develop an equation for describing the movement
of electrons in an atom. The de Broglie equation giving the wavelength \Lambda=h/mv (where h is the Planck
constant and mv the momentum) represented too simple a picture to match the reality particularly with the
inner atomic orbits where the attractive force of the nucleus would result in a very complex and variable
configuration. Schrodinger eventually succeeded in developing his famous
wave equation. His equation was very
similar to classical equations developed earlier for describing many wave phenomena — sound waves, the
vibrations of a string or electromagnetic waves. In Schrodinger wave equation there is an abstract entity,
called
the wave function and which is symbolized by the Greek letter \psi (psi). When applied to the
hydrogen atom, Schrodinger wave equation yielded all the results of Bohr and de Broglie. However,
despite the considerable predictive success of Schrodinger wave mechanics,
Schrodinger had to overcome certain problems. First problem was how to attach certain physical
meaning to an electron if it (wave function) was nothing but wave. Also he had to show what
exactly represented by the wave function.
Schrodinger worked hardly to account these questions. He tried to visualize electron
as
wave packets made up of many small waves so that these wave packets would behave in the same
way as a particle in classical mechanics. However, these packets were later shown to be unstable.
He interpreted the wave function as a measure of the spread of an electron. But this was also not
acceptable. The interpretation was provided by Max Born. He stated that the wave function for a
hydrogen atom represents each of its physical states and it can be used to calculate the probability
of finding the electron at a certain point in space (
the probability amplitude). What does it mean? It means that if the
wave function is nearly zero at a certain point then the probability of finding the electron there
is extremely small. But where the wave function is large the probability of finding the electron is
very large. The wave mechanics cannot be used to determine the motion of a particle or in other words
its position and velocity at any given moment. The wave equation simply tells us how the wave function
evolves in space and time and the value of the wave function would determine the probability of
finding the electron in a particular point of space.
He published his revolutionary work in a series of papers in 1926. Schrodinger wave equation
was the second theoretical explanation for the movement of electrons in an atom, the first
being Werner Heisenberg matrix mechanics. Schrodinger approach was preferred by many physicists
as it could be visualized. On the other hand Heisenberg approach was strictly mathematical and it
involved such a complex mathematics that it was difficult to understand. Physicists appeared to be
divided into two groups. However, soon Schrodinger showed that the two theories were identical but
expressed differently.
Schrodinger students at Zurich found his lectures extremely stimulating and impressive.
One of his students, who attended his lectures, later recalled: At the beginning he stated the
subject and then gave a review of how one had to approach it, and then he started exposing the
basis in mathematical terms and developed it in front of our eyes. Sometimes he would stop and
with a shy smile confess that he had missed a bifurcation in his mathematical development,
turn back to the critical point and start all over again. This was fascinating to watch and we all
learned a great deal by following his calculations, which he developed without ever looking at
his notes, except at the end, when he compared his work on the blackboard with his notes and
said this is correct. In summertime when it was warm enough we went to the bathing beach on the
Lake of Zurich, sat with our own notes on the grass and watched this lean man in bathing trunks
writing his calculations before us on an improvised blackboard which we had brought along. At the
time few people came to the bathing beach in the morning and those that did watched us from a
discreet distance and wondered what that man was writing on the blackboard.
After the retirement of Max Plank from Berlin University as Professor of Theoretical Physics,
three persons were short-listed for the post: Sommerfeld, Schrodinger and Max Born.
Schrodinger testimonial drawn up for the purpose beautifully summarised his academic achievements
till that time. It said: For some years already he has been favourably known through his versatile,
vigorously powerful, and at the same time very profound style in seeking new physical problems that
interested him and illuminating them through deep and original ideas, with the entire set of techniques w
hich mathematical and physical methods at present provide. He has proved this method of working to be
effective in the treatment of problems in statistical mechanics, the analysis of optical interference,
and the physical theory of colour vision. Recently he has succeeded in an especially daring design
through his ingenious idea for the solution of the former particle mechanics by means of wave mechanics
in the differential equation he has set up for the wave function. Schrodinger himself has already been
able to deduce many consequences from this fortunate discovery, and the new ideas that he has inspired
with it in many fields are even more numerous ... it may be added that in lecturing as in discussions
Schrodinger has a superb style, marked by simplicity and precision, the impressiveness of which is
further emphasized by the temperament of a South German. Sommerfeld was the first choice and when he
declined to leave Munich the offer went to Schrodinger. Even for Schrodinger it was not easy for taking
a decision to leave Zurich. Ioan James has written: Every effort was made to persuade him to stay in
Zurich. The physics students organized a torchlight parade around the university to the courtyard of
his house, where they presented him with a petition. Schrodinger was deeply moved, but in the end it
was a personal appeal from Planck that persuaded him to accept the Berlin offer; as the result of doing so
he automatically became a German national. Before taking up the appointment at Berlin, Schrodinger traveled to
Brussels to attend the Solvay physics conferences. This time the topic was electrons and photons. Schrodinger was
invited to deliver one of the prestigious lectures. He took this opportunity to elaborate on his
wave mechanics. His views caused considerable debate. Born and Heisenberg attacked it quite
vehemently.
Schrodinger joined the Berlin University on October 01, 1927, where he became a colleague of
Albert Einstein. The course given by him at the Berlin University was considered the best among the
science courses at the University. His style of lecturing was informal. He lectured without notes
while many professors at the University practically read their lectures. His dress was also quite
informal compared to other professors. He was elected to the Berlin Academy of Science at the age
of forty-two. He happened to be youngest member of this august body.
Like many other scientists Schrodinger had to leave Germany after the Nazis seized power.
The Nazis had no problems with Schrodinger but it was Schrodinger who did not like policies
pursued by the Nazis. In fact Schrodinger disgust for the Nazis was so strong that he was
prepared to leave Germany. Initially Scgrodinger thought the Nazi madness will pass over within a
couple of years but soon he realized that the Nazis are going to stay in power for a long time.
Finally Schrodinger left Germany for Oxford. It was possible for intervention of
Frederick Alexander Lindemann (1886-1957), the head of the physics department at
Oxford University and a close friend of Winston Churchill who could persuade
Magdalen College, Oxford, to offer Schrodinger a Fellowship. Lindemann had visited Germany
in the spring of 1933 to try to arrange positions in England for some young Jewish scientists
from Germany. Schrodinger appointment at Magdalen was to be supplemented by a research
appointment in industry so that his income became comparable to that of an Oxford professor.
The confirmation of his appointment was accompanied by the news that he had just been
awarded Nobel Prize in physics, jointly with Paul Dirac. Schrodinger reached Oxford on
November 04, 1933. Lindemann and other tried their best to make Schrodinger stay at
Oxford comfortable. However, Schrodinger was not satisfied with his status at Oxford.
He had received an offer of a permanent position at the Institute of Advanced Studies at
Princeton during his visit there in the spring of 1934 for giving an invited lecture.
However, finally Schrodinger did not accept the offer.
In 1935 Schrodinger’s published a three-part essay on The present situation in quantum mechanics.
It was in this essay the much talked about Schrodinger cat paradox appears. This paradox was a
thought experiment, where a cat in a closed box either lived or died according to whether a
quantum event occurred or not. Schrodinger appointment at Oxford was extended for another
two years. But he did not stay there. He left for his own country Austria to take up an appointment
at the University of Graz. While waiting for the official confirmation of his appointment at
Graz he received an offer of a professorship at Edinburgh. However, the necessary permission
for permanent British residence did not come before the official confirmation came from Graz.
He finally moved to Graz where he was given a full professorship and also an honorary professorship
at Vienna.
While working at Graz, Schrodinger was hoping that eventually he would get an appointment at Vienna.
But this did not happen. In 1938, the Nazis extended their anti-Semitic policies pursued in Germany
to Austria. The newly appointed Nazi Rector of the University of Graz persuaded Schrodinger
to make a repentant confession. The confession began as follows: In the midst of the
exultant joy which is pervading our country, there also stand today those who indeed partake
fully of this joy but not without deep shame because until the end they had not understood the right
course. And it continued in more or less in the same vein. The confession duly appeared in the press.
Many of his friends thought that Schrodinger could write such a confession only under pressure.
But there was no pressure.
Afterwards Schrodinger, of course, always regretted his decision to write such a confession.
Explaining the reason for writing such a confession to Einstein, Schrodinger wrote:
I wanted to remain free — and could not do so without great duplicity.
Schrodinger attended the celebration of the eightieth birthday of Max Plank, where he was warmly
welcomed. But he was no longer acceptable to the Nazi authorities because they did not forget
the insult he caused to them by fleeing from Berlin in 1933. His so-called repentant confession
was of no use. First he was dismissed from his honorary position at Vienna and then on
August 26, 1938 he was also dismissed from his regular post at Graz. The reason cited for his
dismissal was his political unreliability. The official in Vienna, whom Schrodinger consulted,
advised him to get a job in industry. They also told him that he will not be allowed to leave
the country. Schrodinger immediately realized the danger of staying in Austria. So he hurriedly
left for Italy. They had no time even to take their belongings with them. They boarded the train
to Rome with a few suitcases. Schrodingers were received at the station in Italy by Enrico Fermi,
who also lent them some money. From Rome Schrodinger wrote to the Irish statesman
Eamon De Valera (1882-1975), then President of the League of Nations
(predecessor of the United Nations). Schrodinger met De Valera at Geneva.
De Valera offered Schrodinger a position at the Institute of Advanced Studies that he was trying to
set up at Dublin. De Valera also advised Schrodinger to leave Italy at the earliest and go for
Ireland or England, as according to him the war was imminent. Schrodinger accepted De Valera’s
offer of appointment at the proposed Institute at Dublin. However, he did not directly
proceed to Dublin. Instead he went back to Oxford, where he received an offer of one
year visiting professorship at the University of Ghent in Belgium. At Ghent he wrote a
significant paper on the expanding universe. From Ghent Schrodinger alongwith his family went to Oxford.
Lindemann and others who had earlier welcomed Schrodingers at
Oxford was no longer ready to welcome them again. Now Schrodingers were classed as enemy aliens.
But Lindemann made it possible for Schrodingers to reach Dublin in October 1939. Schrodinger
adjusted well in the new environs and under his leadership the Institute of Advanced Studies of
Dublin became an important centre of theoretical physics. He remained in Dublin until he
retired in 1956.
At the beginning of his stay at Dublin, Schrodinger studied electromagnetic theory and
relativity and began to publish on unified field theory. As we know Einstein was also working
on the same problem at the similarly named Princeton University. In 1947 Schrodinger believed
that he had a real breakthrough in his efforts toward creating unified field theory. Schrodinger
was so excited about his new theory that he decided to present it to the Irish Academy without
examining it critically. Schrodinger announcement was widely publicized in the media as an
epoch-making discovery. However, after seeing Einstein comments Schrodinger realized his folly.
He was really devastated by the episode. It was certainly a great embarrassment. After this debacle
Schrodinger turned to philosophy. His study of Greek science and philosophy is summarised in
Nature and the Greeks, which was published in 1954.
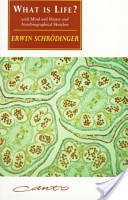
Schrodinger most important contribution at the Dublin Institute was his book called
What is Life?.
This was the result of a series of lectures given at the Institute in 1943. The book was published
in 1944. It is regarded as one of the most important scientific writings of the twentieth century.
Francois Ducheseneau wrote: As a contribution to the Dublin Institute series of public lectures,
Schrodinger, who was an engaging speaker, delivered several in February 1943 under the
title: What is Life? In these popular scientific lectures Schrodinger, who had only a very
slight knowledge of the literature on the physical bases of life, dragged his audience into
and then out of a series of blind alleys, leaving them at the end just about where he began.
Nonetheless these lectures, printed the following year, achieved an immediate and great reputation
with both physicists and biologists, and rank still today as one of the most overrated scientific
writings of the twentieth century. The book influenced a good many talented young physicists
particularly those who were disillusioned by the destruction caused by atom bombs in Japan and
wanted no part in atomic physics. Schrodinger showed these physicists a discipline, which was
free from military applications and at the same time very significant and largely unexplored.
The book represented the transfer of new concepts of physics into biology.
Schrodinger presented a determinist vision of the role of genes. He wrote: In calling the structure
of the chromosome fibers a code-script we mean that the all-penetrating mind, once conceived by
Laplace, to which every causal connection lay immediately open, could tell from their structure
whether the egg would develop, under suitable conditions, into a black cock or into a speckled hen,
into a fly or a maize plant, a rhododendron, a beetle, a mouse or a woman.
It was Schrodinger who first used the word
code to describe the role of gene.
He also observed that with the molecular picture of the gene it is no longer inconceivable
that the miniature should precisely correspond with a highly complicated and specified plan of
development.” The book with such passages, written with more insight than that contained in most
contemporary biochemical works inspired a generation of scientists to look for such a code and
which was eventually found. The book helped to shape the discipline that we call today molecular
biology. Michel Morange wrote: Schrodinger book was a remarkable success. Many of the founders of
molecular biology claimed that it played an important role in their decision to turn to biology.
Gunther Stent, a geneticist (and a historian of genetics), has argued that for the new biologists
it played a role like that of Uncle Tom Cabin. Schrodinger presented the new results of genetics
in a lively, the book has lost none of its seductiveness: its clarity and simply make it a pleasure
to read.
In 1955, Schrodinger returned to Vienna. On his arrival he was treated as a celebrity.
He was appointed to a special professorship at the University of Vienna. Though he retired from
the university in 1958, he continued to be an emeritus professor till his death. In Vienna he
wrote his last book describing his metaphysical views.
Schrodinger died on January 04, 1961. Commenting on Schrodinger personal traits his
biographer Walter Moore wrote: Schrodinger was a passionate man, a poetic man, and the
fire of his genius would be kindled by the intellectual tension arising from the desperate situation
of the old quantum theory…It seems also that psychological stress, particularly that associated with
intense love affairs, helped rather than hindred his scientific creativity.
1. Erwin Schrodinger. What is Life?: With Mind and Matter and Autobiographical Sketches.
(Cambridge University Press, 1992);
2. William T. Scott. Erwin Schroedinger: An Introduction to his Writings. University of Massachusetts Press. 1967. 175pp.
3. Dr. Subodh Mahanti.
Erwin Schrodinger. The Founder of Quantum Wave Mechanics.
4. C. Cohen-Tannoudji, B. Diu, F. Laloe: Quantum Mechanics. Two Vols. (Wiley, 1977);
5. J. J. Sakurai: Modern Quantum Mechanics. Revised Edition (Addison Wesley, 1994);
6. B. H. Brandsden, C. J. Joachain: Quantum Mechanics. 2nd edition (Prentice Hall, 2000);
7. D. I. BLOKHINTSEV, Quantum Mechanics, Dordrecht, Reidel, Netherlands: Kluwer Academic Publishers. 1964;
8. Jagdish Mehra. Erwin Schroedinger and the Rise of Wave Mechanics. (New York: Springer-Verlag. 1987) 392pp;
9. V. V. Raman, P. Forman: Why was it Schroedinger who developed de Broglie ideas?
Historical Studies in the Physical Sciences 1 (1969), 291;
10. L. Wessels: Schroedinger route to wave mechanics. Studies in the History and Philosophy of
Science. 10 (1977), 311;
11. H. Kragh: Erwin Schroedinger and the wave equation: the crucial phase. Centaurus. 26 (1982),
154;
12. M. Jammer: The Conceptual Development of Quantum Mechanics. (Baltimore: Johns Hopkins
University Press, 1966);
13. J. Mehra and H. Rechenberg: The Historical Development of Quantum Theory. (New York:
Springer, vols. 1-6);
14. Schrodinger, Centenary Celebration of a Polymath.
Editor Clive William Kilmister,
(CUP Archive, 1987); ISBN 0521340179, 9780521340175;
15. Walter J. Moore, Schrodinger: Life and Thought (Cambridge University Press, 1992);
16. Michel Bitbol. Schroedinger's Philosophy of Quantum Mechanics. (Dordrecht,
Netherlands: Kluwer Academic Publishers. 1996), 285pp.
17. W. L. Reiter and J. Yngvason, (eds.),
Erwin Schrodinger - 50 Years After (European Mathematical Society, 2013).
===========================================================================
PDF FILE OF THIS PAGE: SCHRODINGER_ERWIN